|
|
|

|
Electrochemistry
Copyright
D. Herrick |
 |
| |
20 Questions from past exams.
Practice for speed. Aim
for 2 minutes per problem.
|
|
General
Information:
*Faraday
constant: F
= 96,500 Coulomb / mol
*Gas
constant:
R = 8.314 J / mol-deg
*Volt conversion:
1 V = 1 J / Coulomb
*The symbol E denotes EMF.
*All reactions are at 25C
= 298K.
|
| 1. |
Given
the half-reactions
Sn2+(aq) + 2e–
 Sn(s) , Eo =
-0.136 V
Sn(s) , Eo =
-0.136 V
AgCl(s) + e–
 Ag(s) + Cl–(aq)
, Eo = 0.222 V
Ag(s) + Cl–(aq)
, Eo = 0.222 V
calculate standard-state EMF (Eo) for an
electrochemical cell based on the reaction
Sn(s) + 2AgCl(s)  2Ag(s) + 2Cl–(aq) + Sn2+(aq)
2Ag(s) + 2Cl–(aq) + Sn2+(aq) |
|
| A)
0.580V |
B)
0.358 |
C)
0.086
|
D)
-0.358
|
E)
-0.580
|
|
| 2. |
In
a working electrochemical cell, reduction occurs at the |
|
| A) |
anode |
| B) |
membrane
barrier |
| C) |
catalyst |
| D) |
acidic
electrode |
| E) |
cathode |
|
| 3. |
Which
statement is FALSE? |
|
| A) |
The
species that gains electrons in an electrochemical
reaction is reduced. |
| B) |
By
convention, the half-reaction
2H+(aq) + 2e–
 H2(g)
H2(g)
has a standard EMF of Eo = 0. |
| C) |
The
species that loses electrons in an electrochemical
reaction is oxidized. |
| D) |
The
standard state of O2(g) has P = 1 atm. |
| E) |
The
standard state of Cu2+(aq) has [Cu2+]
= 1 M. |
| F) |
The
reaction quotient Q satisfies Q = 1 when reactants and
products have their standard-state concentrations or
pressures. |
| G) |
The
reaction quotient satisfies Q = K when all reactants and
products have their equilibrium concentrations or
pressures. |
| H) |
K
in the preceding answer (G) is the equilibrium constant
expression. |
| I) |
The
free-energy change for a chemical reaction with
reactants and products at arbitrary concentrations or
pressures is given by
 G
= G
=  Go +
RT ln(Q) = RT ln(Q/K) . Go +
RT ln(Q) = RT ln(Q/K) .
This means the spontaneous direction of reaction
favors formation of more products when Q < K, and
more reactants when Q > K. Some students prefer to
write these as K > Q and K < Q so that the symbols
> and < point in the direction of spontaneous
reaction. |
| J) |
The
Nernst equation for the EMF (E, in Volts) generated by
an electrochemical cell at 25C is derived from the
free-energy change to be
E = Eo - (0.0592/ne)
log(Q)
where "log" refers to
base 10 (ex: log(100) = 2). |
| K) |
ne
in the preceding answer (J) is the number of mols of
electrons transferred in the balanced
oxidation-reduction reaction. |
| L) |
The
EMF of a spontaneous electrochemical reaction is
positive. |
| M) |
The
maximum possible work from a voltaic cell is obtained
when the current is drawn very slowly. |
| N) |
Since
the EMF of an electrochemical reaction satisfies E = 0
at equilibrium, the equilibrium constant for the
reaction at 25C can be determined from the Nernst
equation in the form
log(K) = ne
Eo / 0.0592
when Eo is given in
Volts.
|
| O) |
none
of the above |
|
| 4. |
Find
the value of ne for a voltaic cell based on
oxidation-reduction half-reactions for the combustion
reaction:
CH4
+ 2 O2  CO2
+ 2 H2O CO2
+ 2 H2O
|
|
| A) |
ne=
1 |
F) |
ne=
6 |
| B) |
ne=
2 |
G) |
ne=
7 |
| C) |
ne=
3 |
H) |
ne=
8 |
| D) |
ne=
4 |
I) |
ne=
9 |
| E) |
ne=
5 |
J) |
ne=
10 |
|
| 5. |
In
a working electrochemical cell oxidation occurs at the |
|
| A) |
anode |
| B) |
surface
of the liquid |
| C) |
catalyst |
| D) |
acidic
electrode |
| E) |
cathode |
|
| 6. |
The
role of Cu2+ in the electron transfer
reaction
Zn
+ Cu2+  Zn2+ + Cu
is
Zn2+ + Cu
is
|
|
| A) |
oxidizing
agent |
| B) |
spectator
ion |
| C) |
reducing
agent |
| D) |
catalyst |
|
| 7. |
The
FALSE statement about a working electrochemical cell is: |
|
| A) |
The
anode is the electrode at which oxidation of chemical
species occurs. |
| B) |
Cations
and anions--not electrons--conduct the electrical
current in solution. |
| C) |
The
EMF of a voltaic cell is independent of the temperature. |
| D) |
Aqueous
ions with positive charges migrate toward the
cathode. |
|
| 8. |
The
strongest oxidizing agent is: |
|
| A) |
Fe2+ |
E) |
Sn2+ |
| B) |
Cr3+ |
F) |
Mg2+ |
| C) |
Sn |
G) |
Fe |
| D) |
Cr |
H) |
Mg |
|
| 9. |
The
strongest reducing agent is: |
|
| A) |
Fe2+ |
E) |
Sn2+ |
| B) |
Cr3+ |
F) |
Mg2+ |
| C) |
Sn |
G) |
Fe |
| D) |
Cr |
H) |
Mg |
|
| 10. |
Find
the standard EMF (Eo) of the reaction
3Sn(s) + 2Cr3+(aq)
 2Cr(s) + 3Sn2+(aq)
2Cr(s) + 3Sn2+(aq) |
|
| A) |
-1.76
V |
F) |
0.44 |
| B) |
-1.20 |
G) |
0.60 |
| C) |
-0.88 |
H) |
0.88 |
| D) |
-0.60 |
I) |
1.20 |
| E) |
-0.44 |
J) |
1.76 |
|
| 11. |
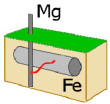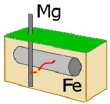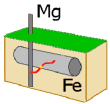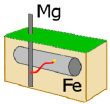 Magnesium
metal attached to a piece of iron acts as a
"sacrificial anode" that inhibits rusting because Mg loses electrons more readily than Fe. Calculate
Eo for the reaction Magnesium
metal attached to a piece of iron acts as a
"sacrificial anode" that inhibits rusting because Mg loses electrons more readily than Fe. Calculate
Eo for the reaction
Mg(s) + Fe2+(aq)  Mg2+(aq)
+ Fe(s)
Mg2+(aq)
+ Fe(s)
|
|
| A) |
2.79
V |
| B) |
1.97 |
| C) |
0 |
| D) |
-1.97 |
| E) |
-2.79 |
|
| 12. |
When
Cu is obtained by electrolysis from CuSO4 ,
the minimum number of coulombs required to produce 1.25
mol of copper is |
|
| A) |
38,600 |
| B) |
77,200 |
| C) |
60,312 |
| D) |
120,625 |
| E) |
241,250 |
|
| 13. |
When
Al is obtained by electrolysis from Al2O3
, the number of coulombs required to produce 1.00 mol of
aluminum is |
|
| A) |
32,167 |
| B) |
64,333 |
| C) |
193,000 |
| D) |
289,500 |
| E) |
579,000 |
|
| 14. |
Calculate
Eo for the reaction
2Al(s) + 3Fe2+(aq)  2Al3+(aq)
+ 3Fe(s)
2Al3+(aq)
+ 3Fe(s)
given
Fe2+(aq) + 2e–
 Fe(s) ,
Eo = -0.41 V
Fe(s) ,
Eo = -0.41 V
Al3+(aq) + 3e–
 Al(s), Eo = -1.66 V
Al(s), Eo = -1.66 V |
|
| A) 2.09V |
B)
1.25 |
C)
zero |
D)
-1.25 |
E)
-2.09 |
|
| 15. |
What
is ne in the Nernst equation for the reaction
in Question 14? |
|
| 16. |
Use
the Nernst equation to determine the emf for a voltaic cell
based on the reaction
Cl2(g, 1atm) + 2I–
(aq, 2.00M)  2Cl– (aq, 0.02M) + I2(s), 2Cl– (aq, 0.02M) + I2(s),
Eo = 0.83 V |
|
| A) 1.07V |
B)
0.59 |
C)
0.71 |
D)
1.66 |
E)
0.95 |
|
| 17. |
Use
the Nernst equation to determine the emf for a voltaic
cell based on the
reaction
Cr(s) + 3 Ag+(0.00010
M)  Cr3+(0.010 M) + 3 Ag(s),
Cr3+(0.010 M) + 3 Ag(s),
Eo = 1.540 V |
|
| A) 1.34V |
B)
1.29 |
C)
1.74 |
D)
1.54 |
E)
1.88 |
|
| 18. |
Find
the equilibrium constant for the reaction
O2(g) + 4H+(aq)
+ 4Fe2+(aq)  4Fe3+(aq)
+ 2 H2O(l ) ,
4Fe3+(aq)
+ 2 H2O(l ) ,
Eo = 0.460V |
|
| A) |
5.9
× 107 |
| B) |
3.6
× 1016 |
| C) |
8.1
× 10-32 |
| D) |
1.2
× 1031 |
| E) |
8.3
× 1028 |
|
| 19. |
Calculate
the EMF generated by a voltaic cell operating on the
reaction
Zn(s) + 2H+(aq,
pH=3)  Zn2+(aq, 1M) + H2(g, 1 atm),
Zn2+(aq, 1M) + H2(g, 1 atm),
Eo = 0.76 V |
|
| A) 1.12V |
B)
0.46 |
C)
0.67 |
D)
0.58 |
E)
0.94 |
|
| 20. |
Find
the equilibrium constant of the reaction
Fe(s) + Cd2+(aq)  Fe2+(aq) + Cd(s),
Fe2+(aq) + Cd(s),
Eo = 0.0400V |
|
| A)
22.5 |
B)
4.7 |
C)
0.045 |
D)
0.21 |
E)
1.00 |
|
|
|
|