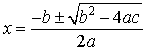CH223
- 11:00
EXAM-1A
Practice
|
Information: |
|
gas
constant: R = 0.08206 L·atm/mole·K
= 8.314 J/mole·K
Avogadro's number: NA
= 6.022 ´
1023/mol
van't Hoff equation: ln(K2/K1)= -(DH/R)(1/T2
-1/T1)
normal freezing point of water:
0.00°C = 273.15K
water ionization constant (25°C): Kw = 1.00 ´
10-14
pressure: 1 atm = 760 mmHg
= 760 torr
exp(x) = ex,
ln(ex) = x, log(10x)
= x
quadratic equation:
ax2 + bx + c = 0, |
|
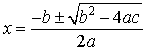 |
Multiple
choice: select
one answer for each question:
1.
Find the concentration of B in an equilibrium gas mixture that has [C] = 4.61 ´
10-2 M:
B(g)  2C(g)
, Kc =
5.38 ´
10-3
2C(g)
, Kc =
5.38 ´
10-3
|
a) |
8.5
´
10-2 M |
|
b) |
9.2 ´
10-1 M |
|
c) |
3.1 ´
10-3 M |
|
d) |
4.0 ´
10-1 M |
|
e) |
1.9 ´
10-2 M |
|
|
|
2.
The relationship between Kc and Kp for the reaction NO(g)
+ ½ O2(g)  NO2(g) at 477ºC is
NO2(g) at 477ºC is
|
a) |
Kc = 483
Kp |
|
b) |
Kc
= 7.85 Kp |
|
c) |
Kc = 15.6
Kp |
|
d) |
Kc = 2.07
´
10-3 Kp |
|
e) |
Kc =
Kp |
|
|
|
3.
If Kc = 0.145 for A2 + 2B 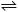 2AB,
then for AB
2AB,
then for AB 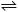 B + ½ A2, Kc would equal
B + ½ A2, Kc would equal
|
|
|
|
a) |
0.381 |
|
b) |
2.63 |
|
c) |
0.145 |
|
d) |
-0.145 |
|
e) |
6.90 |
|
|
|
4. Predict the reaction direction when
[CS2] = [H2] = [CH4] = [H2S]
= 0.125 M:
CS2(g) + 4 H2(g)
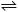 CH4(g)
+ 2 H2S(g) ,
Kc = 28
CH4(g)
+ 2 H2S(g) ,
Kc = 28
|
|
|
|
|
a) |
reactants 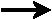 products
products |
|
|
b) |
no net reaction, system is in equilibrium |
|
|
c) |
reactants 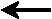 products
products |
|
|
|
|
|
5. Which of these equilibria would be affected by pressure changes
at constant temperature?
1. FeO(s) + CO(g) 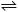 Fe(s)
+ CO2(g)
Fe(s)
+ CO2(g)
2. CaCO3(s) 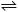 CaO(s) + CO2(g)
CaO(s) + CO2(g)
3. 2Mg(s) + CO2(g) 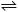 2MgO(s) + C(s)
2MgO(s) + C(s)
|
|
|
|
a) |
1 only |
|
b) |
2 only |
|
c) |
3 only |
|
d) |
1 and 2 only |
|
e) |
2 and 3 only |
|
|
|
6. The
reaction 5CO(g) + I2O5(g) 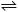 I2(g) + 5CO2(g) is exothermic. The yield
could be increased by
I2(g) + 5CO2(g) is exothermic. The yield
could be increased by
|
|
|
|
a) |
increasing
the pressure |
|
b) |
decreasing
the pressure |
|
c) |
increasing
the temperature |
|
d) |
decreasing
the temperature |
|
e) |
decreasing
the volume of the reaction vessel |
|
|
|
7.
The symbol Kb(HCO3-) is the equilibrium
constant for which reaction?
8.
The pH of a solution that has [H3O+]
= 5.12 × 10-3 M is:
|
|
|
|
|
a) |
6.4 |
|
b) |
2.3 |
|
c) |
5.1 |
|
d) |
3.0 |
|
|
|
9.
The Bronsted concept of acids and bases says the conjugate base of NH3
would be
|
|
|
|
|
a) |
OH - |
|
b) |
NH4OH |
|
c) |
NH2- |
|
d) |
NH4+ |
|
|
|
10.
What is [OH-] in a solution that has [H3O+] =
3.16 × 10-5 M?
|
|
|
|
a) |
3.16 × 10-8 M |
|
b) |
3.16
× 10-11 M |
|
c) |
3.16
× 10-9 M |
|
d) |
3.16
× 10-10 M |
|
|
|
11.
The
concentration of H3O+ in 0.44 M
hypobromous acid (HBrO, Ka = 2.3 ´
10-9) is:
|
|
|
|
a) |
2.6 ´
10-4 M |
|
b) |
3.2 ´
10-5 M |
|
c) |
1.4 ´
10-6 M |
|
d) |
2.1 ´
10-5 M |
|
|
|
12.
What
is the concentration of cyanide ion CN-
in 0.003 M HCN (Ka = 6.2 × 10-10)?
|
|
|
|
a) |
1.4 ´
10-6 M |
|
b) |
6.2 ´
10-10 M |
|
c) |
8.4 ´
10-7 M |
|
d) |
2.5 ´
10-5 M |
|
|
|
13.
The
pH of 0.033 M NH3 (Kb = 1.8 × 10-5) is
|
|
|
|
|
a) |
11.3 |
|
b) |
9.25 |
|
c) |
10.9 |
|
d) |
12.8 |
|
|
|
14. Which
solution has the lowest pH?
|
|
|
|
a) |
0.10 M
HNO2 |
|
b) |
0.10 M
HNO3 |
|
c) |
0.10 M
KNO2 |
|
d) |
0.10 M
KNO3 |
|
|
|
15.
The correct order of increasing base strength (weaker < stronger) is:
|
|
|
|
|
a) |
BrO3-
< BrO2-
< BrO-
|
|
|
b) |
BrO3-
< BrO-
< BrO2- |
|
|
c) |
BrO2-
< BrO-
< BrO3- |
|
|
d) |
BrO-
< BrO2-
< BrO3- |
|
|
|
|
|
16.
Fe3+ in the reaction
Fe3+ + 6 H2O 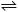 Fe(H2O)63+ is best described as a
Fe(H2O)63+ is best described as a
|
|
|
|
a) |
Bronsted
acid |
|
b) |
Bronsted
base |
|
c) |
Lewis
acid |
|
d) |
Lewis
base |
|
e) |
Arrhenius
acid |
|
|
|
17.
How does the acid equilibrium shift after some sodium acetate salt (CH3COONa
) is added to a solution of acetic acid (CH3COOH, Ka = 1.8
´
10-5)?
18.
Use
the acid constants for HCN (Ka = 6 ´ 10-10) and HClO (Ka = 3 ´
10-8) to calculate Kc for
ClO-(aq)
+ HCN(aq) 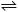 CN-(aq)
+ HClO(aq)
CN-(aq)
+ HClO(aq)
|
|
|
|
a) |
2 ´ 10-3 |
|
b) |
2 ´
10-2 |
|
c) |
2 ´
10-4 |
|
d) |
2 ´
103 |
|
e) |
2 ´
1012 |
|
|
|
19.
A diprotic acid H2A has values of Ka1 = 1.0 ´
10-6 and Ka2 = 1.0 ´
10-10.
What is [A2-]
in a 0.10 M solution of H2A?
|
|
|
|
a) |
0.10
M |
|
b) |
0.20
M |
|
c) |
3.2 ´
10-4 |
|
d) |
3.2 ´
10-6 |
|
e) |
1.0 ´
10-10
|
|
|
|
20.
Calculate the degree of
ionization in 0.72 M acid HA (Ka = 0.12).
HINT: use the quadratic equation.
|
|
|
|
a) |
33% |
|
b) |
50% |
|
c) |
75% |
|
d) |
25% |
|
e) |
67%
|
|
|
|

| KEY: |
 |