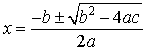CH223
- 11:00
EXAM-1B
Practice
|
Information: |
|
gas
constant: R = 0.08206 L·atm/mole·K
= 8.314 J/mole·K
Avogadro's number: NA
= 6.022 ´
1023/mol
van't Hoff equation: ln(K2/K1)= -(DH/R)(1/T2
-1/T1)
normal freezing point of water:
0.00°C = 273.15K
water ionization constant (25°C): Kw = 1.00 ´
10-14
pressure: 1 atm = 760 mmHg
= 760 torr
exp(x) = ex,
ln(ex) = x, log(10x)
= x
quadratic equation:
ax2 + bx + c = 0, |
|
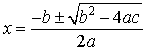 |
Multiple
choice: select
one answer for each question:
1. Calculate
Kc for A  2B
+ C if [A] = 0.125 M, [B] = 0.862 M and
[C] = 0.642 M at equilibrium.
2B
+ C if [A] = 0.125 M, [B] = 0.862 M and
[C] = 0.642 M at equilibrium.
|
a) |
2.86 |
|
b) |
4.43 |
|
c) |
3.82
|
|
d) |
5.36 |
|
|
|
2.
An aqueous solution is basic if the condition ______ is satisfied.
|
a) |
[H3O+]
< [OH-] |
|
b) |
[H3O+]
= [OH-] |
|
c) |
[H3O+]
> [OH-] |
|
|
|
3. For
which of the following values of Kc does the reaction go the most
toward completion?
|
|
|
|
a) |
1012 |
|
b) |
1025
|
|
c) |
10-25 |
|
d) |
10-12 |
|
|
|
4.
The relationship between Kc and Kp
for the reaction A(s)  B(g)
+ C(g) at 25°C is
B(g)
+ C(g) at 25°C is
|
|
|
|
|
a) |
Kc
= 1.67 ´
10-3 Kp
|
|
|
b) |
Kc
= 24.5 Kp |
|
|
c) |
Kc
= 4.09 ´
10-2 Kp |
|
|
d) |
Kc
= 1.63 ´
10-7
Kp |
|
|
e) |
Kc
= 598 Kp |
|
|
|
|
|
5. The
pH of 5.26 × 10-6 M HCl is
|
|
|
|
a) |
6.41 |
|
b) |
5.15 |
|
c) |
3.63 |
|
d) |
5.28 |
|
e) |
4.94 |
|
|
|
6.
Kp = 0.17 for A(g)  2B(g).
Which way does the
reaction go when PA = 0.30 atm and PB = 0.25 atm?
2B(g).
Which way does the
reaction go when PA = 0.30 atm and PB = 0.25 atm?
|
|
|
|
a) |
A
 2B
2B |
|
b) |
A
 2B
2B |
|
c) |
no
change, system is at equilibrium |
|
|
|
7.
If
Kc = 0.962 for A + 2B  3C,
then Kc for 6C
3C,
then Kc for 6C  4B
+ 2A would equal
4B
+ 2A would equal
|
|
|
|
|
a) |
1.03 |
|
b) |
0.98 |
|
c) |
1.04 |
|
d) |
0.93 |
|
e) |
1.08 |
|
|
|
8.
HClO
has Ka = 3 ´
10-8. Determine Kb
for ClO-.
|
|
|
|
|
a) |
3
´
10-6
|
|
b) |
3
´
10-22 |
|
c) |
3
´
10-7 |
|
d) |
3
´
10-9 |
|
e) |
3
´
10-15 |
|
|
|
9.
Calculate [B] in an equilibrium mixture
that has [A] = 0.500 M, [C] = 0.125
M:
A(g)  B(g)
+ C(g) , Kc
= 4.38 ´
10-5
B(g)
+ C(g) , Kc
= 4.38 ´
10-5
|
|
|
|
|
a) |
5.6
´
10-5 M |
|
b) |
2.7
´
10-6
M |
|
c) |
7.0
´
10-4 M |
|
d) |
1.8
´
10-4 M |
|
e) |
1.1
´
10-5 M |
|
|
|
10.
The
reaction A(g)  B(g)
+ C(g) is endothermic.
The highest yield of products is obtained at
B(g)
+ C(g) is endothermic.
The highest yield of products is obtained at
|
|
|
|
a) |
low
temperature, high pressure |
|
b) |
high
temperature, high pressure |
|
c) |
low
temperature, low pressure |
|
d) |
high
temperature, low pressure |
|
|
|
11.
At
equilibrium the forward (1) and reverse (-1) rates of the reaction 2A  B
satisfy
B
satisfy
|
|
|
|
a) |
Rate1
= 2 Rate-1 |
|
b) |
2
Rate1 = Rate-1 |
|
c) |
Rate1
= 4 Rate-1 |
|
d) |
4
Rate1 = Rate-1 |
|
e) |
Rate1
= Rate-1 |
|
|
|
12.
The pH of a 2.48 ×
10-3 M solution of the soluble salt Ba(OH)2
is
|
|
|
|
a) |
11.7 |
|
b) |
9.30 |
|
c) |
10.6 |
|
d) |
9.52 |
|
e) |
8.48 |
|
|
|
13.
The concentration
of F-
in 0.65 M HF (Ka = 6.80 ´
10-4) is:
|
|
|
|
|
a) |
3.2
´
10-2 M |
|
b) |
2.1
´
10-2 M |
|
c) |
6.8
´
10-3 M |
|
d) |
2.4
´
10-2
M |
|
e) |
0.65
M |
|
|
|
14. Which
statements are TRUE?
1 = O2-
is the conjugate base of the hydroxide ion.
2 = Water is amphoteric.
3 = H3PO4 is the conjugate acid of H2PO4-
|
|
|
|
a) |
1 only |
|
b) |
2 only |
|
c) |
3 only |
|
d) |
1 and 3
only |
|
e) |
1, 2,
and 3 |
|
|
|
15.
The
concentration of hydronium ion in 0.125 M NH3 (Kb = 1.8 ×
10-5) is
|
|
|
|
|
a) |
5.42
´
10-12 M |
|
|
b) |
6.13
´
10-12 M |
|
|
c) |
4.80
´
10-11 M |
|
|
d) |
6.67
´
10-12 M |
|
|
e) |
7.25
´
10-12 M |
|
|
|
|
|
16.
The correct order of increasing
base strength in water is expected to be
|
|
|
|
a) |
F-
< Cl-
< Br- |
|
b) |
Cl-
< F-
< Br- |
|
c) |
Br-
< Cl-
< F- |
|
d) |
F-
< Br-
< Cl- |
|
e) |
Br-
< F-
< Cl- |
|
|
|
17.
NH3 in the reaction Ag+
+ 2 NH3  Ag(NH3)2+
is a ______
Ag(NH3)2+
is a ______
1 = Arrhenius base
2 = Bronsted-Lowry base
3 = Lewis base
|
|
|
|
|
a) |
1
only |
|
b) |
2
only |
|
c) |
3
only |
|
d) |
2
and 3 only |
|
e) |
1,
2 and 3 |
|
|
|
18.
Sulfurous
acid H2SO3 is a weak diprotic oxoacid.
The concentrations of species in a 0.25 M solution
of H2SO3 are expected to satisfy
|
|
|
|
a) |
[H2SO3]
> [HSO3-
]
> [SO32-
] |
|
b) |
[H2SO3]
< [HSO3-
]
> [SO32-
] |
|
c) |
[H2SO3]
> [HSO3-
]
< [SO32-
] |
|
d) |
[H2SO3]
< [HSO3-
]
< [SO32-
] |
|
e) |
[H2SO3]
= [HSO3-
]
> [SO32-
] |
|
|
|
19.
Use the acid constants for H2S
(pKa1 = 7, pKa2 = 17) and HIO (pKa = 11)
to determine Kc
for
IO-(aq)
+ HS-(aq)
 HIO(aq)
+ S2-(aq)
HIO(aq)
+ S2-(aq)
|
|
|
|
a) |
1
´
10-28 |
|
b) |
1
´
10-9 |
|
c) |
1
´
10-14 |
|
d) |
1
´
10-8 |
|
e) |
1
´
10-6
|
|
|
|
20.
Calculate the
degree of ionization of HIO3 in 0.16 M HIO3 (Ka
= 0.24). HINT:
use quadratic equation.
|
|
|
|
a) |
78% |
|
b) |
69% |
|
c) |
58% |
|
d) |
64% |
|
e) |
73%
|
|
|
|

| KEY: |
 |