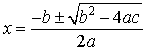CH223
- 11:00
EXAM-2A
Practice
|
Information: |
|
gas
constant: R = 0.08206 L·atm/mole·K
= 8.314 J/mole·K
Avogadro's number: NA
= 6.022 ´
1023/mol
Q-dependence of free energy change: DG
= DGo
+ RT ln(Q)
normal freezing point of water:
0°C = 273K
water ionization constant (25°C): Kw = 1.00 ´
10-14
pressure: 1 atm = 760 mmHg
= 760 torr
log conversion: ln(x) =
2.303 log(x)
quadratic equation:
ax2 + bx + c = 0, |
|
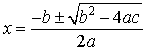 |
Multiple
choice: select
one answer for each question:
1.
The pH of a buffer solution that consists of 0.58 M boric acid (H3BO3
, pKa = 9.24) and 0.27 M sodium borate (NaH2BO3)
is:
|
|
|
|
a) |
8.70 |
|
b) |
9.34 |
|
c) |
8.46 |
|
d) |
9.57 |
|
e) |
8.91 |
|
|
|
2.
The best criterion for the spontaneity
of a chemical reaction at constant T and P is the sign of
|
|
|
|
a) |
DH |
|
b) |
DH° |
|
c) |
TDS |
|
d) |
DG |
|
e) |
DG° |
|
|
|
3.
Find
DG°
for the reaction at 298K: I2(g) + Cl2(g)  2
ICl(g), DH°
= -26.9 kJ,
DS°
= 11.3 J/K
2
ICl(g), DH°
= -26.9 kJ,
DS°
= 11.3 J/K
|
|
|
|
a) |
102
kJ |
|
b) |
-30.3
kJ |
|
c) |
50.6
kJ |
|
d) |
+18.4
kJ |
|
e) |
-50.6
kJ |
|
|
|
4. The
solubility of PbSO4 (Ksp = 1 ´
10-8
)
in water is
|
|
|
|
|
a) |
1
´
10-4
M |
|
|
b) |
1
´
10-7
M |
|
|
c) |
1
´
10-5
M |
|
|
d) |
1
´
10-8
M |
|
|
e) |
1
´
10-6
M |
|
|
|
|
|
5. In which of the following reactions will
DS°
be positive?
|
|
1.
N2O4(g) ®
2NO2(g)
2. CaO(s) + CO2(g)
®
CaCO3(s)
3.
sublimation
of CO2 |
|
|
|
|
a) |
1 only |
|
b) |
2 only |
|
c) |
1 and 2 only |
|
d) |
1 and 3 only |
|
e) |
1, 2 and 3 |
|
|
|
6. The
equation associated with Kf for the complex ion Ag(NH3)2+
is
|
|
|
|
a) |
Ag(NH3)2+(aq)
 Ag+(aq)
+ 2NH3(aq) Ag+(aq)
+ 2NH3(aq) |
|
b) |
Ag+(aq)
+ 2NH3(aq)
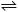 Ag(NH3)2+(aq) Ag(NH3)2+(aq) |
|
c) |
Ag(NH3)2+(aq)
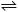 Ag+(g)
+ 2NH3(g) Ag+(g)
+ 2NH3(g) |
|
d) |
Ag+(g)
+ 2NH3(g) 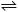 Ag(NH3)2+(aq) Ag(NH3)2+(aq) |
|
e) |
Ag+(aq)
+ N2(g) + 3H2(g) 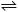 Ag(NH3)2+(aq) Ag(NH3)2+(aq) |
|
|
|
7.
The pH after mixing 0.00020 mole of HCl
and 0.00018 mole of NaOH in 1.000 L of solution is:
|
|
|
|
|
a) |
4.70 |
|
b) |
5.00 |
|
c) |
5.30 |
|
d) |
5.60 |
|
e) |
9.00 |
|
|
|
8.
A chemical equilibrium that
favors reactants at high temperature and products at low temperature has
|
|
|
|
|
a) |
DHo
> 0, DSo
> 0 |
|
b) |
DHo
> 0, DSo
< 0 |
|
c) |
DHo
< 0, DSo
< 0 |
|
d) |
DHo
< 0, DSo
> 0 |
|
e) |
none of the above |
|
|
|
9.
Calculate
the equilibrium constant Kp for the reaction at 500K:
|
|
|
Cu(s)
+ H2O(g) 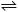 CuO(s)
+ H2(g),
DG°500K
= 110.6 kJ CuO(s)
+ H2(g),
DG°500K
= 110.6 kJ |
|
|
|
|
a) |
2.4
´
10-13 |
|
b) |
5.6
´
10-12 |
|
c) |
0.97 |
|
d) |
3.5
´
10-11 |
|
e) |
2.8
´
10-12 |
|
|
|
10.
Calculate
the solubility of Cu(OH)2 (Ksp =1 ´10-20)
in a solution buffered at pH = 6.00.
|
|
|
|
a) |
1
´10-6
M |
|
b) |
1
´10-14
M |
|
c) |
1
´10-8
M |
|
d) |
1
´10-4
M |
|
e) |
1
´10-10
M |
|
|
|
| Questions 11-15: Titrate 64 mL of 0.200M weak acid HA (Ka
= 1 ´
10-8 ) with 0.200M NaOH. |
11.
The
equilibrium concentrations at the equivalence point satisfy
|
|
|
|
a) |
[HA]
<
[A-
]
|
|
b) |
[HA] =
[A-
]
|
|
c) |
[HA]
>
[A-
]
|
|
|
|
12. The
volume of NaOH needed to reach the buffer point is
|
|
|
|
a) |
64
mL |
|
b) |
16
mL |
|
c) |
32
mL |
|
d) |
48
mL |
|
e) |
72 mL |
|
|
|
13.
The
pH at the buffer point is
14. At
pH = 7 the quotient [A-
] / [HA] is
|
|
|
|
a) |
0.01 |
|
b) |
0.10 |
|
c) |
1 |
|
d) |
10 |
|
e) |
100 |
|
|
|
15.
The pH at the equivalence point
satisfies
|
|
|
|
|
a) |
pH < 7 |
|
|
b) |
pH = 7 |
|
|
c) |
pH > 7 |
|
|
|
|
|
| Questions 16-20: Titrate 60 mL of 0.50 M acid H2A
(Ka1 = 1 ´
10-3
, Ka2 = 1 ´
10-9
) with 0.50 M NaOH . |
16. The
volume of NaOH needed to reach the first equivalence point is:
|
|
|
|
a) |
90
mL |
|
b) |
30
mL |
|
c) |
75
mL |
|
d) |
60
mL |
|
e) |
45
mL |
|
|
|
17.
The pH at the
first equivalence point is:
18.
Equilibrium
concentrations at the first equivalence point satisfy:
|
|
|
|
a) |
[HA-
]
> [A2-
]
> [H2A] |
|
b) |
[H2A]
= [HA-
]
> [A2-
] |
|
c) |
[HA-
]
> [A2-
]
= [H2A] |
|
d) |
[A2-
]
= [HA-
]
> [H2A] |
|
e) |
[HA-
]
> [H2A] > [A2-
] |
|
|
|
19.
Equilibrium
concentrations at pH = 4 satisfy:
|
|
|
|
a) |
[HA-
]
> [H2A] > [A2-
] |
|
b) |
[A2-
]
= [HA-
]
> [H2A] |
|
c) |
[HA-
]
> [A2-
]
= [H2A] |
|
d) |
[H2A]
= [HA-
]
> [A2-
] |
|
e) |
[HA-
]
> [A2-
]
> [H2A]
|
|
|
|
20.
Equilibrium concentrations at
the second buffer point satisfy:
|
|
|
|
a) |
[A2-
]
> [HA-
]
> [H2A] |
|
b) |
[A2-
]
= [HA-
]
> [H2A] |
|
c) |
[HA-
]
> [H2A] > [A2-
] |
|
d) |
[HA-
]
> [A2-
]
= [H2A] |
|
e) |
[H2A]
= [HA-
]
> [A2-
]
|
|
|
|

| KEY: |
 |