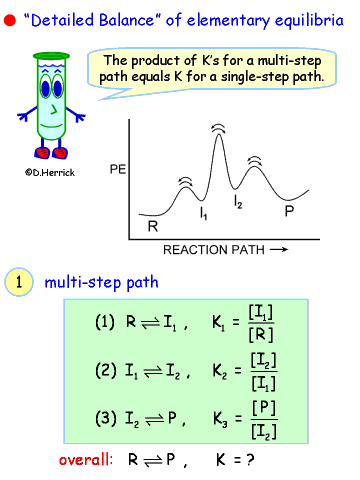
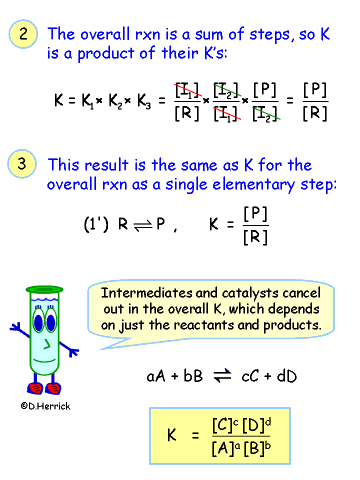
| |
Note |
| |
|
| |
Aqua
Regia. A mixture of the two
strong acids HCl and HNO3 that dissolves
"royal" metals such as gold that are otherwise difficult to put into solution for reactions
or purification. The power to dissolve gold is related to the
redox reaction
Au + 4H+ + 4Cl -
+ NO3-
 AuCl4- + NO
+ 2H2O
AuCl4- + NO
+ 2H2O
in which the chloride ion facilitates
oxidation of Au to the auric ion Au3+. We’ll be
looking into oxidation-reduction reactions and electrochemistry in
Chapter 21. When the solution containing excess HCl is evaporated
it crystallizes out hydrated tetrachlorauric acid, HAuCl4.4H2O.
The related salt NaAuCl4.2H2O has been
used in toning photographic prints. Aqua regia is commonly
used in laboratories simply to clean things.
|
| |
Temperature
Dependence of Kw.
The pH of neutral water at room temperature is approximately
7.00, but this value changes at higher or lower
temperatures. The water ionization reaction
2H2O  H3O+ + OH-
H3O+ + OH-
is endothermic, so LeChatelier's
principle says the equilibrium shifts to increase the hydronium ion
concentration (and hence decrease pKw and the neutral
pH) as the temperature is raised:
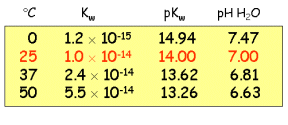
You can find a more extensive tabulation in the CRC
Handbook of Chemistry and Physics.
|
 |
Acid-Base Distribution Diagrams.
Diagrams for the distribution of acid-base species from a
multiprotic acid HnA
may be determined as a function of pH by expressing
ratios of species in terms of the acid equilibrium constants K1,
K2,.... Here are the final
expressions for the mole fraction of each species. You can
use these to make accurate plots of their relative amounts in
aqueous solution.
1)
monoprotic acid HA (K1)
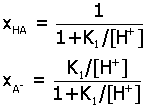
2) diprotic
acid H2A (K1, K2)
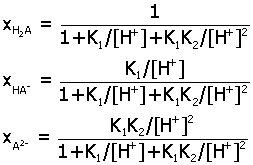
3)
triprotic acid H3A (K1, K2, K3)
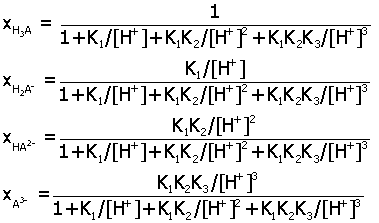
|
 |
Role
of Kw in pH of weak acid or base solutions. See frames 6-9 of the April 9 lecture.
Kw may be neglected when the pH is less than 6 or greater than
8.
|
| |
|
| |
|
| |
Links
|
| |
Profiles: Linus
Pauling, Maria
Mayer (&
picture), |
| |
Nobel
Prizes in Chemistry
|
| |
amino
acid structures and pKa
values |
| |
accurate
values of physical constants:
http://physics.nist.gov/PhysRefData/contents.html |
| |
National
Chemistry Week |
| |
Ann
Herrick: Books for Kids & Teens |
| |
|